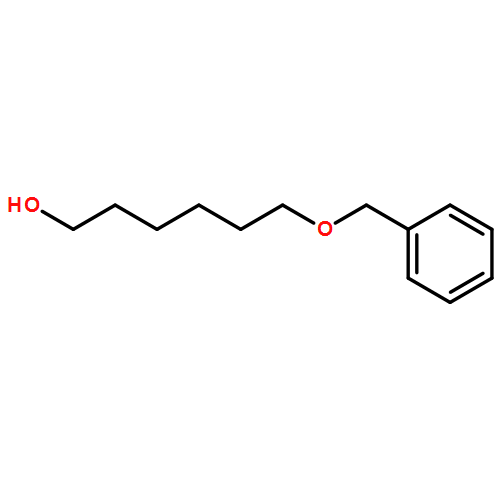
A facile and efficient protocol for the oxidative deprotection of benzyl-type ethers has been developed. The reaction was performed with catalytic amounts of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and tert-butyl nitrite (TBN) under atmospheric pressure of O2. Under the optimal reaction conditions, a variety of p-methoxybenzyl (PMB), p-phenylbenzyl (PPB), and benzyl (Bn) ethers can be deprotected to their corresponding alcohols in excellent conversions and selectivities.A facile and efficient protocol for the oxidative deprotection of benzyl-type ethers has been developed. The reaction was performed with catalytic amounts of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and tert-butyl nitrite (TBN) under atmospheric pressure of O2. Under the optimal reaction conditions, a variety of PMB, PPB, and Bn ethers can be deprotected to their corresponding alcohols in excellent conversions and selectivities.