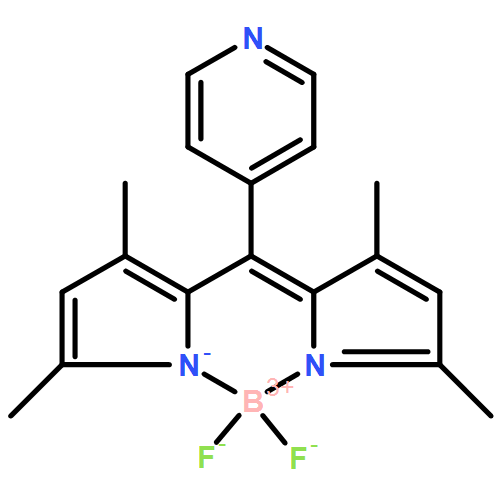
Co-reporter: Juergen Bartelmess, Walter W. Weare and Roger D. Sommer
pp: 14883-14891
Publication Date(Web):30 Aug 2013
DOI: 10.1039/C3DT51849C
We report the synthesis of four boron–dipyrromethene (BODIPY) sensitized cobaloxime complexes as structural models of light-driven proton reduction catalysts. The BODIPY chromophore is covalently linked to the cobaloxime via a pyridine molecule in the meso-position of the BODIPY. Electron withdrawing or donating substituents on the pyridine (e.g. chloro- or methyl-groups) allow a comparison of the influence of these substituents on cobaloxime–BODIPY interactions. This includes altering the overall stability of the complexes and tuning the catalytically relevant CoII/III redox couple over a range of 220 mV, which will assist in the design of future cobaloxime–chromophore complexes. All complexes were characterized using electrochemistry, electronic absorption and fluorescence spectroscopy. Additionally, we present crystal structures of the four new BODIPY–cobaloxime complexes and elucidate the influence of the structural modifications. We found that these compounds produce sub-stoichiometric quantities of hydrogen under standard photon-driven hydrogen evolving conditions.
Co-reporter: Juergen Bartelmess, Walter W. Weare and Roger D. Sommer
pp: NaN14891-14891
Publication Date(Web):2013/08/30
DOI: 10.1039/C3DT51849C
We report the synthesis of four boron–dipyrromethene (BODIPY) sensitized cobaloxime complexes as structural models of light-driven proton reduction catalysts. The BODIPY chromophore is covalently linked to the cobaloxime via a pyridine molecule in the meso-position of the BODIPY. Electron withdrawing or donating substituents on the pyridine (e.g. chloro- or methyl-groups) allow a comparison of the influence of these substituents on cobaloxime–BODIPY interactions. This includes altering the overall stability of the complexes and tuning the catalytically relevant CoII/III redox couple over a range of 220 mV, which will assist in the design of future cobaloxime–chromophore complexes. All complexes were characterized using electrochemistry, electronic absorption and fluorescence spectroscopy. Additionally, we present crystal structures of the four new BODIPY–cobaloxime complexes and elucidate the influence of the structural modifications. We found that these compounds produce sub-stoichiometric quantities of hydrogen under standard photon-driven hydrogen evolving conditions.