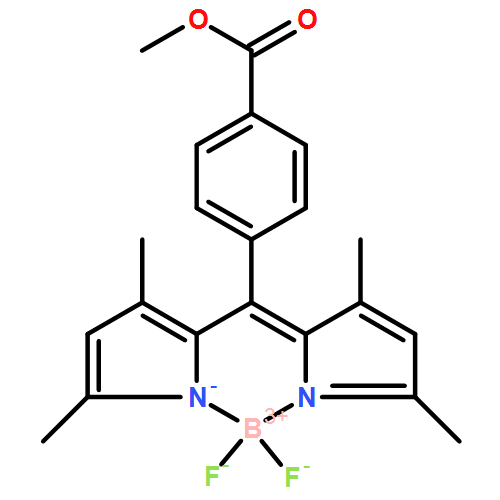
Co-reporter: Geng-Geng Luo, Jiu-Xu Xia, Kai Fang, Qing-Hua Zhao, Ji-Huai Wu and Jing-Cao Dai
pp: 16268-16271
Publication Date(Web):24 Sep 2013
DOI: 10.1039/C3DT52325J
4-Methoxycarbonylphenyl-substituted BODIPY (boron-dipyrromethene) dye gives, in the solid state, three polymorphs. In spite of the absence of any strong intermolecular interactions in all crystalline forms, the three polymorphs show different photoluminescence properties. This behavior highlights the importance of molecular arrangements of the BODIPY moieties in the crystalline state in perturbing their photophysical properties.
Co-reporter: Geng-Geng Luo, Jiu-Xu Xia, Kai Fang, Qing-Hua Zhao, Ji-Huai Wu and Jing-Cao Dai
pp: NaN16271-16271
Publication Date(Web):2013/09/24
DOI: 10.1039/C3DT52325J
4-Methoxycarbonylphenyl-substituted BODIPY (boron-dipyrromethene) dye gives, in the solid state, three polymorphs. In spite of the absence of any strong intermolecular interactions in all crystalline forms, the three polymorphs show different photoluminescence properties. This behavior highlights the importance of molecular arrangements of the BODIPY moieties in the crystalline state in perturbing their photophysical properties.
Co-reporter: Geng-Geng Luo, Hui Lu, Xiao-Long Zhang, Jing-Cao Dai, Ji-Huai Wu and Jia-Jia Wu
pp: 9716-9729
Publication Date(Web):10 Mar 2015
DOI: 10.1039/C5CP00732A
A series of boron dipyrromethene (BODIPY) dyes (B1–B5) having H atoms at 2,6-positions or heavy-atom I at 2-/2,6-positions, and an ortho- or a para-COOH substituted phenyl moiety at the 8-position on the BODIPY core were synthesized and characterized. These organic dyes were applied for investigating the relationship between the BODIPY structure and the effectiveness of homogeneous and heterogeneous visible-light-driven hydrogen production as well as dye-sensitized solar cells (DSSCs). For the homogeneous photocatalytic hydrogen production systems with a cobaloxime catalyst, the efficiency of hydrogen production could be tuned by substituting with heavy atoms and varying carboxyl group orientations of BODIPYs. As a result, B5 containing two I atoms and an ortho-COOH anchoring group was the most active one (TONs = 197). The activity of hydrogen generation followed the order B5 > B3 > B2 > B1 = B4 = 0. An interesting “ortho-position effect” was observed in the present homogeneous systems, i.e., substitution groups were located at the ortho-position and higher hydrogen production activities were obtained. For the heterogeneous hydrogen production systems with a platinized TiO2 catalyst, the effectiveness of hydrogen evolution was highly influenced by the intersystem crossing efficiency, molar absorptivity and positions of the anchoring group of dyes. Thus, B3 having two core iodine atoms and a para-COOH group with TONs of 70 excelled other BODIPYs and the TONs of hydrogen generation showed the trend of B3 > B5 > B2 > B1 = B4 = 0. The results demonstrate that the present photocatalytic H2 production proceeds with higher efficiency and stability in the homogeneity than in the heterogeneity. In the case of DSSCs, the overall cell performance of BODIPY chromophores was highly dependent on both the absence or the presence of iodine atoms on the BODIPY core and –COOH anchoring positions. The B1–TiO2 system showed the best cell performance, because the most effective surface binding mode is allowed with this structure. This is also in contrast with the case of dye-sensitized solar H2 generation, in which B3 was the most efficient chromophore. The differences between dye-sensitized hydrogen-generating systems and DSSCs may be due to rates of electron transfer and the dye aggregation tendency.
Co-reporter: Geng-Geng Luo, Hui Lu, Xiao-Long Zhang, Jing-Cao Dai, Ji-Huai Wu and Jia-Jia Wu
pp: NaN9729-9729
Publication Date(Web):2015/03/10
DOI: 10.1039/C5CP00732A
A series of boron dipyrromethene (BODIPY) dyes (B1–B5) having H atoms at 2,6-positions or heavy-atom I at 2-/2,6-positions, and an ortho- or a para-COOH substituted phenyl moiety at the 8-position on the BODIPY core were synthesized and characterized. These organic dyes were applied for investigating the relationship between the BODIPY structure and the effectiveness of homogeneous and heterogeneous visible-light-driven hydrogen production as well as dye-sensitized solar cells (DSSCs). For the homogeneous photocatalytic hydrogen production systems with a cobaloxime catalyst, the efficiency of hydrogen production could be tuned by substituting with heavy atoms and varying carboxyl group orientations of BODIPYs. As a result, B5 containing two I atoms and an ortho-COOH anchoring group was the most active one (TONs = 197). The activity of hydrogen generation followed the order B5 > B3 > B2 > B1 = B4 = 0. An interesting “ortho-position effect” was observed in the present homogeneous systems, i.e., substitution groups were located at the ortho-position and higher hydrogen production activities were obtained. For the heterogeneous hydrogen production systems with a platinized TiO2 catalyst, the effectiveness of hydrogen evolution was highly influenced by the intersystem crossing efficiency, molar absorptivity and positions of the anchoring group of dyes. Thus, B3 having two core iodine atoms and a para-COOH group with TONs of 70 excelled other BODIPYs and the TONs of hydrogen generation showed the trend of B3 > B5 > B2 > B1 = B4 = 0. The results demonstrate that the present photocatalytic H2 production proceeds with higher efficiency and stability in the homogeneity than in the heterogeneity. In the case of DSSCs, the overall cell performance of BODIPY chromophores was highly dependent on both the absence or the presence of iodine atoms on the BODIPY core and –COOH anchoring positions. The B1–TiO2 system showed the best cell performance, because the most effective surface binding mode is allowed with this structure. This is also in contrast with the case of dye-sensitized solar H2 generation, in which B3 was the most efficient chromophore. The differences between dye-sensitized hydrogen-generating systems and DSSCs may be due to rates of electron transfer and the dye aggregation tendency.