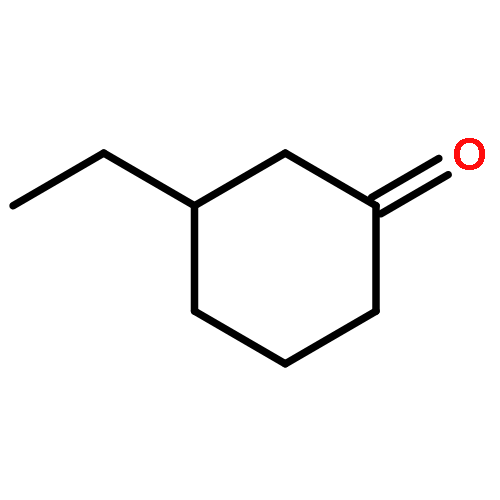
A series of novel chiral diphosphite ligands have been synthesized from d-mannitol derivatives and chlorophosphoric acid diary ester, and were successfully employed in the copper catalyzed enantioselective conjugate addition of organozinc reagents diethylzinc and dimethylzinc to cyclic and acyclic enones. The stereochemically matched combination of d-mannitol and (R)-H8-binaphthyl in ligand 1,2:5,6-di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite-d-mannitol was essential to afford 93% ee for 3-ethylcyclohexanone, 92% ee for 3-ethylcyclopentanone, and 90% ee for 3-ethylcycloheptanone in toluene, using Cu(OTf)2 as a catalytic precursor. The results clearly indicated that the chiral organocopper reagent exhibited high enantioselectivies for cyclic enones bearing different ring sizes. As for the backbone of this type of ligand, it has been demonstrated that 1,2:5,6-di-O-isopropylidene-d-mannitol was more efficient than 1,2:5,6-di-O-cyclohexylidene-d-mannitol. The sense of the enantiodiscrimination was mainly determined by the configuration of the diaryl phosphite moieties in the 1,4-addition of cyclic enones.


1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2

[α]D20=-165 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2

[α]D20=+184 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2

[α]D20=-309 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2

[α]D20=+496 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2

[α]D20=-205 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2

[α]D20=+213 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)