We have developed an organocatalyzed asymmetric cascade sulfa-Michael-aldol reaction between 2-mercaptoindole-3-carbaldehydes and enals, which provides efficient access to the stereocontrolled construction of dihydrothiopyrano[2,3-b]indole skeletons. Under the catalysis of chiral diphenylprolinol TMS ether, the reactions ran smoothly to give the corresponding synthetically useful and pharmaceutically valuable dihydrothiopyrano[2,3-b]indoles in high yields and with 64–96% ee.


(R)-N-tert-Butoxycarbonyl-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H21NO3S[α]D20 = +183.2 (c 0.5, CH2Cl2)93% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

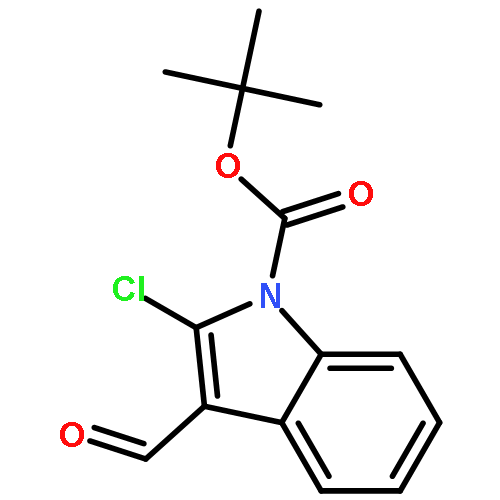
(R)-N-tert-Ethoxycarbonyl-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC21H17NO3S[α]D20 = +216.3 (c 0.5, CH2Cl2)67% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-Tosyl-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC25H19NO3S2[α]D20 = +190.6 (c 0.5, CH2Cl2)73% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-(4-fluorophenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20FNO3S[α]D20 = +173.0 (c 0.5, CH2Cl2)85% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(S)-N-tert-Butoxycarbonyl-2-(2-fluorophenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20FNO3S[α]D20 = +239.5 (c 0.5, CH2Cl2)89% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (S)

(R)-N-tert-Butoxycarbonyl-2-(4-chlorophenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20ClNO3S[α]D20 = +260.5 (c 0.5, CH2Cl2)81% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-(3-chlorophenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20ClNO3S[α]D20 = +173.8 (c 0.5, CH2Cl2)83% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-(4-bromophenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20BrNO3S[α]D20 = +163.9 (c 0.5, CH2Cl2)66% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(S)-N-tert-Butoxycarbonyl-2-(2-bromophenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20BrNO3S[α]D20 = +304.8 (c 0.5, CH2Cl2)75% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-(3-trifluoromethylphenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC24H20F3NO3S[α]D20 = +183.4 (c 0.5, CH2Cl2)96% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-(2-methoxyphenyl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC24H23NO4S[α]D20 = +330.2 (c 0.5, CH2Cl2)78% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(S)-N-tert-Butoxycarbonyl-2-(furan-2-yl)-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC21H19NO4S[α]D20 = +153.2 (c 0.5, CH2Cl2)78% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (S)

(R)-N-tert-Butoxycarbonyl-2-propyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC20H23NO3S[α]D20 = +93.8 (c 0.5, CH2Cl2)64% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-isopropyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC20H23NO3S[α]D20 = +80.3 (c 0.5, CH2Cl2)75% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-2-pentyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC22H27NO3S[α]D20 = +140.8 (c 0.5, CH2Cl2)77% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-6-fluoro-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20FNO3S[α]D20 = +152.8 (c 0.5, CH2Cl2)93% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-6-chloro-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20ClNO3S[α]D20 = +88.0 (c 0.5, CH2Cl2)87% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-7-chloro-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20ClNO3S[α]D20 = +93.6 (c 0.5, CH2Cl2)73% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)

(R)-N-tert-Butoxycarbonyl-6-bromo-2-phenyl-2,9-dihydrothiopyrano[2,3-b]indole-3-carbaldehydeC23H20BrNO3S[α]D20 = +95.6 (c 0.5, CH2Cl2)89% eeSource of chirality: Asymmetric organocatalysisAbsolute configuration: (R)