Co-reporter: Sally E. Pusede, Trevor C. VandenBoer, Jennifer G. Murphy, Milos Z. Markovic, Cora J. Young, Patrick R. Veres, James M. Roberts, Rebecca A. Washenfelder, Steven S. Brown, Xinrong Ren, Catalina Tsai, Jochen Stutz, William H. Brune, Eleanor C. Browne, Paul J. Wooldridge, Ashley R. Graham, Robin Weber, Allen H. Goldstein, Sebastien Dusanter, Stephen M. Griffith, Philip S. Stevens, Barry L. Lefer, and Ronald C. Cohen
pp: 12774-12781
Publication Date(Web):October 5, 2015
DOI: 10.1021/acs.est.5b02511
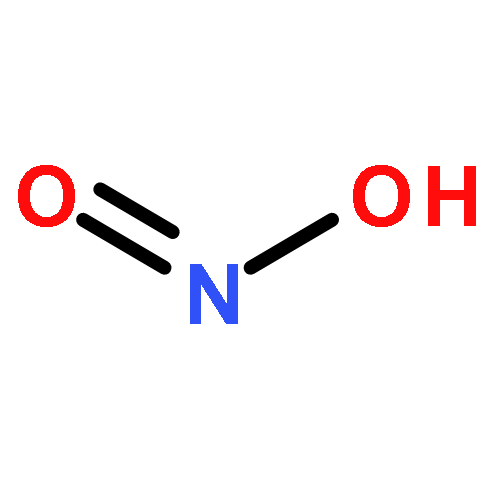
Recent observations suggest a large and unknown daytime source of nitrous acid (HONO) to the atmosphere. Multiple mechanisms have been proposed, many of which involve chemistry that reduces nitrogen dioxide (NO2) on some time scale. To examine the NO2 dependence of the daytime HONO source, we compare weekday and weekend measurements of NO2 and HONO in two U.S. cities. We find that daytime HONO does not increase proportionally to increases in same-day NO2, i.e., the local NO2 concentration at that time and several hours earlier. We discuss various published HONO formation pathways in the context of this constraint.