Co-reporter: Yao-Qing Shen, Florence Bonnot, Erin M. Imsand, Jordan M. RoseFigura, Kimmen Sjölander, and Judith P. Klinman
pp:
Publication Date(Web):February 10, 2012
DOI: 10.1021/bi201763d
Pyrroloquinoline quinone (PQQ) is a small, redox active molecule that serves as a cofactor for several bacterial dehydrogenases, introducing pathways for carbon utilization that confer a growth advantage. Early studies had implicated a ribosomally translated peptide as the substrate for PQQ production. This study presents a sequence- and structure-based analysis of the components of the pqq operon. We find the necessary components for PQQ production are present in 126 prokaryotes, most of which are Gram-negative and a number of which are pathogens. A total of five gene products, PqqA, PqqB, PqqC, PqqD, and PqqE, are identified as being obligatory for PQQ production. Three of the gene products in the pqq operon, PqqB, PqqC, and PqqE, are members of large protein superfamilies. By combining evolutionary conservation patterns with information from three-dimensional structures, we are able to differentiate the gene products involved in PQQ biosynthesis from those with divergent functions. The observed persistence of a conserved gene order within analyzed operons strongly suggests a role for protein–protein interactions in the course of cofactor biosynthesis. These studies propose previously unidentified roles for several of the gene products, as well as identifying possible new targets for antibiotic design and application.
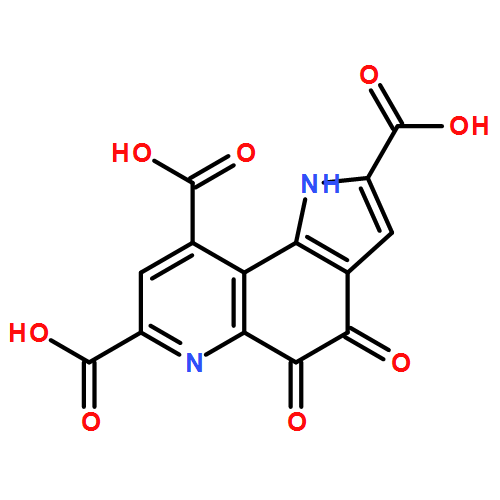
Co-reporter: Florence Bonnot, Anthony T. Iavarone, and Judith P. Klinman
pp:
Publication Date(Web):May 29, 2013
DOI: 10.1021/bi4003315
The final step of the biosynthesis of prokaryotic cofactor PQQ is catalyzed by PqqC, a cofactorless oxidase that brings about a ring closure and overall eight-electron oxidation of its substrate. Time-dependent acid quenching and subsequent high-performance liquid chromatography separation and mass spectrometric analyses of reaction mixtures were performed to correlate the structures of intermediates with previously observed UV–visible signatures. The reaction is composed of four stepwise oxidations: three steps use O2 as the two-electron acceptor, and the fourth uses hydrogen peroxide (H2O2). The chemical nature of the intermediates, the stoichiometry of the reaction, and their dependence on the oxygen concentration indicate that the third oxidation uses the product, H2O2, from the preceding step to produce water. The last oxidation step can also be studied separately and is a reaction between O2 and PQQH2 trapped in the active site. This oxidation is approximately 10 times slower than the reoxidation of PQQH2 in solution. From the order of the four oxidation steps and their sensitivity to O2 concentration, we propose a progressive closure of the active site as the enzyme proceeds through its catalytic cycle.