Co-reporter: Rajat S. Das, Milinda Samaraweera, Martha Morton, José A. Gascón, and Ashis K. Basu
pp: 2451
Publication Date(Web):October 1, 2012
DOI: 10.1021/tx300302a
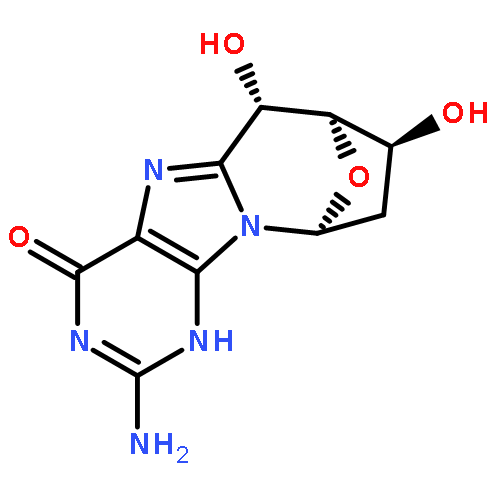
8,5′-Cyclopurine deoxynucleosides are unique tandem lesions containing an additional covalent bond between the base and the sugar. These mutagenic and genotoxic lesions are repaired only by nucleotide excision repair. The N-glycosidic (or C1′-N9) bond of 2′-deoxyguanosine (dG) derivatives is usually susceptible to acid hydrolysis, but even after cleavage of this bond of the cyclopurine lesions, the base would remain attached to the sugar. Here, the stability of the N-glycosidic bond and the products formed by formic acid hydrolysis of (5′S)-8,5′-cyclo-2′-deoxyguanosine (S-cdG) were investigated. For comparison, the stability of the N-glycosidic bond of 8,5′-cyclo-2′,5′-dideoxyguanosine (ddcdG), 8-methyl-2′-deoxyguanosine (8-Me-dG), 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-Oxo-dG), and dG was also studied. In various acid conditions, S-cdG and ddcdG exhibited similar stability to hydrolysis. Likewise, 8-Me-dG and dG showed comparable stability, but the half-lives of the cyclic dG lesions were at least 5-fold higher than those of dG or 8-Me-dG. NMR studies were carried out to investigate the products formed after the cleavage of the C1′-N9 bond. 2-Deoxyribose generated α and β anomers of deoxyribopyranose and deoxyribopyranose oligomers following acid treatment. S-cdG gave α- and β-deoxyribopyranose linked guanine as the major products, but α and β anomers of deoxyribofuranose linked guanine and other products were also detected. The N-glycosidic bond of 8-Oxo-dG was found exceptionally stable in acid. Computational studies determined that both the protonation of the N7 atom and the rate constant in the bond breaking step control the overall kinetics of hydrolysis, but both varied for the molecules studied indicating a delicate balance between the two steps. Nevertheless, the computational approach successfully predicted the trend observed experimentally. For 8-Oxo-dG, the low pKa of O8 and N3 prevented appreciable protonation, making the free energy for N-glycosidic bond cleavage in the subsequent step very high.