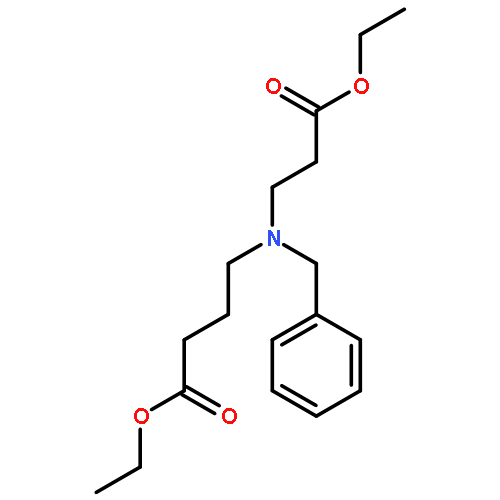
The in situ synthesis and subsequent reactions of macrocylic imine 2 are reported. The imine was trapped with cyanotrimethylsilane to give α-amino nitrile aza-[13]-macrodiolides in a 1:1 ratio of diastereomers. A crystal structure of the syn α-cyano nitrile diastereomer, 7a, provided insights into the lack of selectivity in reactions of 2 relative to macrocyclic alkene 1. Reactions to functionalize the syn diastereomer 7a are also reported.