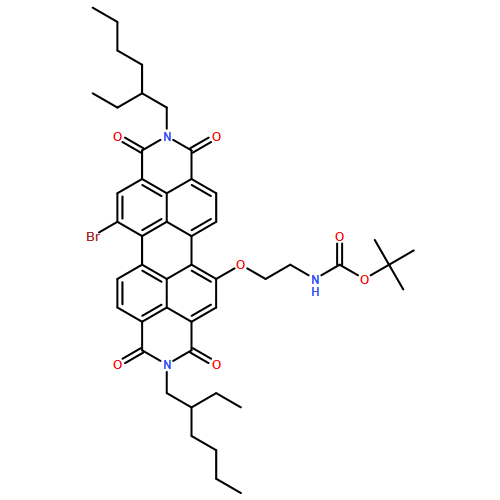
In this Letter, we report a facile approach to synthesize unsymmetrical 1-bromo-7-alkoxyl perylene diimides by the nucleophilic substitution of one of the two 1,7-dibromo units with an alkyl alcohol using K2CO3 as the base. A further replacement of another bromo, for example, by using 4-hydroxylpyridine resulted in unsymmetrical functionalizations at the 1,7-positions. The optical properties of the unsymmetrical PDI derivative were reported and compared with those of the symmetrical derivative.In this Letter, we report a facile approach to synthesize unsymmetrical 1-bromo-7-alkoxyl perylene diimides by the nucleophilic substitution of one of the two 1,7-dibromo units with an alkyl alcohol using K2CO3 as the base. A further replacement of another bromo, for example, by using 4-hydroxylpyridine resulted in unsymmetrical functionalizations at the 1,7-positions. The optical properties of the unsymmetrical PDI derivative were reported and compared with those of the symmetrical derivative.