A novel catalytic system for the hydrogenation of dimethyl itaconate has been developed by using rhodium–diphosphite complexes. These chiral diphosphite ligands were derived from glucopyranoside, d-mannitol derivatives, and binaphthyl or H8-binaphthyl phosphochloridites. The ligands based on the methyl 3,6-anhydro-α-d-glucopyranoside backbone and (R)- and (S)-binaphthol and/or (R)- and (S)-2,2′-dihydroxy-5,5′,6,6′,7,7′,8,8′-octahydro-1,1′-binaphthol gave almost complete conversion of the dimethyl itaconate and both enantiomers of dimethyl 2-methylsuccinate with excellent enantioselectivities. The stereochemically matched combination of methyl 3,6-anhydro-α-d-glucopyranoside and H8-(S)-binaphthyl in ligand 2,4-bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl]-phosphite} methyl 3,6-anhydro-α-d-glucopyranoside was essential to afford dimethyl 2-methylsuccinate with up to 98% ee. The sense of the enantioselectivity of products was predominantly determined by the configuration of the biaryl moieties of the ligands. An initial screening of [Rh(cod)2]BF4 with these ligands in the hydrogenation of (E)-2-(3-butoxy-4-methoxybenzylidene)-3-methylbutanoic acid was carried out. Good enantioselectivity (75% ee) and low yield for (R)-2-(3-butoxy-4-methoxybenzyl)-3-methylbutanoic acid were obtained.


2,4-Bis{[(R)-1,1′-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H34O9P2

[α]D20=-321 (c 0.2, CH2Cl2)Source of chirality: d-glucose

H8− BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Ra,Ra)

2,4-Bis{[(S)-1,1′-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H34O9P2

[α]D20=+502 (c 0.2, CH2Cl2)Source of chirality: D-glucose and BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Sa,Sa)

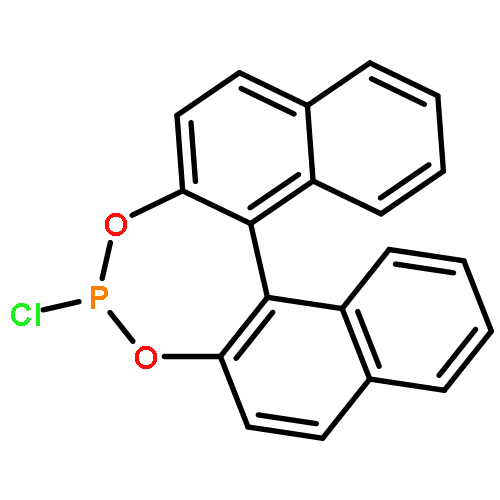
2,4-Bis{[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H50O9P2

[α]D20=-126 (c 0.2, CH2Cl2)Source of chirality: d-glucose and H8-BINOLAbsolute configuration: (1S, 2R, 3S, 4R, 5R, Ra, Ra)

2,4-Bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H50O9P2

[α]D20=+309 (c 0.2, CH2Cl2)Source of chirality: d-glucose and H8-BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Sa,Sa)

2,4-Bis{[(R)-1,1′-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H36O9P2

[α]D20=-451 (c 0.2, CH2Cl2)Source of chirality: d-glucose and BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Ra,Ra)

2,4-Bis{[(S)-1,1′-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H36O9P2

[α]D20=+417 (c 0.2, CH2Cl2)Source of chirality: d-glucose and BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Sa,Sa)

2,4-Bis{[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H52O9P2

[α]D20=-255 (c 0.4, CH2Cl2)Source of chirality: d-glucose and H8-BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Ra,Ra)

2,4-Bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H52O9P2

[α]D20=+230 (c 0.4, CH2Cl2)Source of chirality: d-glucose and H8-BINOLAbsolute configuration: (1S,2R,3S,4R,5R,Sa,Sa)

C58H52O10P21,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitol

[α]D20=-365 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H52O10P2

[α]D20=+437 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)

1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2

[α]D20=-165 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2

[α]D20=+184 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2

[α]D20=-309 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2

[α]D20=+496 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2

[α]D20=-205 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Ra,Ra)

1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2

[α]D20=+213 (c 0.2, CH2Cl2)Source of chirality: d-mannitol and H8-BINOLAbsolute configuration: (2R,3R,4R,5R,Sa,Sa)

 1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2
1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2 1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2
1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2
 2,4-Bis{[(R)-1,1′-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H34O9P2
2,4-Bis{[(R)-1,1′-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H34O9P2 2,4-Bis{[(S)-1,1′-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H34O9P2
2,4-Bis{[(S)-1,1′-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H34O9P2 2,4-Bis{[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H50O9P2
2,4-Bis{[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H50O9P2 2,4-Bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H50O9P2
2,4-Bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-methyl 3,6-anhydro-α-d-glucopyranosideC47H50O9P2 2,4-Bis{[(R)-1,1′-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H36O9P2
2,4-Bis{[(R)-1,1′-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H36O9P2 2,4-Bis{[(S)-1,1′-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H36O9P2
2,4-Bis{[(S)-1,1′-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H36O9P2 2,4-Bis{[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H52O9P2
2,4-Bis{[(R)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H52O9P2 2,4-Bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H52O9P2
2,4-Bis{[(S)-1,1′-H8-binaphthyl-2,2′-diyl] phosphite}-phenyl 3,6-anhydro-β-d-glucopyranosideC52H52O9P2 C58H52O10P21,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitol
C58H52O10P21,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitol 1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H52O10P2
1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H52O10P2 1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2
1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2 1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2
1,2:5,6-Di-O-cyclohexylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC58H68O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H44O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(R)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2 1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2
1,2:5,6-Di-O-isopropylidene-3,4-bis[(S)-1,1′-H8-binaphthyl-2,2′-diyl]phosphite-d-mannitolC52H60O10P2