Co-reporter: Liang Cui, Wenjie Ye, Erin G. Prestwich, John S. Wishnok, Koli Taghizadeh, Peter C. Dedon, and Steven R. Tannenbaum
pp: 195
Publication Date(Web):November 9, 2012
DOI: 10.1021/tx300294d
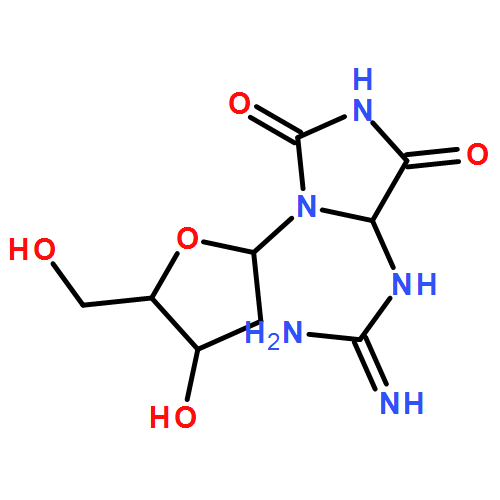
Oxidative damage to DNA has many origins, including irradiation, inflammation, and oxidative stress, but the chemistries are not the same. The most oxidizable base in DNA is 2-deoxyguanosine (dG), and the primary oxidation products are 8-oxodG and 2-amino-imidazolone. The latter rapidly converts to 2,2-diamino-oxazolone (Ox), and 8-oxodG is further oxidized to spiroiminodihydantoin (Sp) and guanidinohydantoin (Gh). In this study, we have examined the dose–response relationship for the formation of the above four products arising in calf thymus DNA exposed to gamma irradiation, photoactivated rose bengal, and two sources of peroxynitrite. In order to carry out these experiments, we developed a chromatographic system and synthesized isotopomeric internal standards to enable accurate and precise analysis based upon selected reaction monitoring mass spectrometry. 8-OxodG was the most abundant products in all cases, but its accumulation was highly dependent on the nature of the oxidizing agent and the subsequent conversion to Sp and Gh. Among the other oxidation products, Ox was the most abundant, and Sp was formed in significantly greater yield than Gh.